Wellcome PNS
Wellcome Peripheral Nerve Stimulator
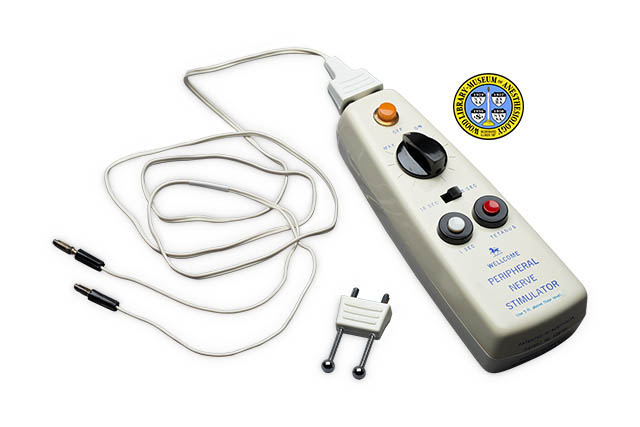
For general anesthesia, muscle relaxants are used to keep the body still during surgery. Muscle relaxants have also been used to control the muscle spasms caused by tetanus. Peripheral nerve stimulators (PNS) are used to determine the extent to which the drug has taken effect. Stimulating a motor nerve with a mild electrical current causes the related muscles to contract. The degree of this response is the most accurate guide to the extent of relaxation, called neuromuscular blockade. The British pharmaceutical firm, Burroughs Wellcome, introduced its first PNS device, the Block-Aid Monitor, in 1964. The Wellcome Peripheral Nerve Stimulator was introduced a few years later. This device could be used either for continuous or intermittent monitoring throughout a surgical procedure. It could also be used for brief applications, such as in the recovery room, or for the treatment of tetanus. The example shown here was donated by the family of anesthesiologist John B. Stetson, M.D. (1927-1993.)
Catalog Record: Wellcome PNS Wellcome PNS
Access Key: aqtc
Accession No.: 1994-06-07-1 D
Title: Wellcome Peripheral Nerve Stimulator / Burroughs Wellcome & Co.
Corporate Author: Burroughs Wellcome and Co.
Publisher: Sydney, New South Wales, Australia : Burroughs Wellcome, [between 1964 and 1993].
Physical Description: 1 peripheral nerve stimulator : plastics, metals ; 175 x 7 x 175 cm.
Subject: Electric Stimulation – instrumentation.
Subject: Monitoring, Physiologic – instrumentation.
Subject: Neuromuscular Monitoring – instrumentation.
Subject: Peripheral Nerve Stimulator.
Subject: Physical Examination – instrumentation.
Note Type: General
Notes: The first year in the range of possible dates of manufacture is based on date of introduction of the first PNS device made by Burroughs Wellcome, the “Block-Aid Monitor”. The earliest published reference to the “Wellcome Peripheral Nerve Stimulator” found by the cataloger is dated 1968, and appears to be an advertisement. The second year in the date range is based on the date of death of the previous owner, John B. Stetson, M.D. (1927-1993.)
Described from the user’s point of view, with the object lying flat. The top of the object is considered that side on which the controls are mounted. The front end is considered that side on which the patent number is printed. The back end is occupied by the receptacle for the probe electrodes.
Note Type: With
Notes: The associated container is a black vinyl pouch with metal zipper, measuring approximately 26.5 x 22 x 10 centimeters. This was not photographed.
Note Type: Citation
Notes: Burroughs Wellcome & Co. Wellcome Peripheral Nerve Stimulator. Sydney, N.S.W., Australia: Burroughs Wellcome (Australia) Ltd., ND.
Note Type: Citation
Notes: Carlson DA, Munson ES. Nerve-stimulator test unit. Anes Analg. July-Augusts, 1970;53(4):629-630.
Note Type: Citation
Notes: Grace’s Guide to British Industrial History website. https://www.gracesguide.co.uk/Burroughs,_Wellcome_and_Co. Accessed February 5, 2018.
Note Type: Citation
Notes: Hudes E, Kwok CL, Eng M. Clinical use of peripheral nerve stimulators in anaesthesia. Canadian Society of Anaesthesiologists Journal. September, 1987;34(5):525-534.
Note Type: Citation
Notes: Katz RL. Comparison of electrical and mechanical recording of spontaneous and evoked muscle activity. Anesthesiology. March-April,1965;26(2):204-211.
Note Type: Citation
Notes: Lippmann M, Fields WA. Burns of the skin caused by a peripheral nerve stimulator. Anesthesiology. January, 1974;40(1):82-84.
Note Type: Citation
Notes: Raj PP, Rosenblatt R, Montgomery SJ. Use of the nerve stimulator for peripheral blocks. Regional Anes and Pain Medicine. April-June, 1980;5(2):14-21.
Note Type: Physical Description
Notes: One peripheral nerve stimulator; The short description gives the maximum depth with the electrical cord attached; The length of the removable cord alone is approximately 155 centimeters; This cord is white, with a white two-prong jack at one end, which can be inserted in the receptacles at the front end of the unit, and two black jacks
The front end is marked: “PATENTED IN AUSTRALIA [new line] PATENT NO. 408766 [new line] BURROUGHS WELLCOME & CO. [new line] (AUSTRALIA) LTD. ROSEBERRY, N. S. W.”; The back end holds two black plastic receptacles; The bottom half is attached by three screws; The bottom has no visible manufacturer’s markings, but has been etched: “J. B. STETSON, M.D.”; A hand-written adhesive label on the bottom reads: “1.5 MILLISEC. DUR. [new line] MAX = 300 Vts; LINEAR [new line] TETANUS = 50 Hz”.
The depth of the unit without either the cord or the probe electrodes is approximately 20 centimeters; The case has the shape of an elongated triangle with slightly rounded sides and ends; The case is made of cream-colored plastic, with all text printed in blue; From top to bottom (that is, from back end to front end), the top of the unit holds a knurled orange plastic button (called the “signal light” in the product literature); Below this is a black plastic dial marked, from left to right: “Max Off On” (called the “on/off switch-voltage control” in the product literature); Straight lines radiate around this dial (these indicate voltage settings); Below this is a black plastic toggle switch marked ” 10 SEC 5 SEC” (called the “twitch frequency switch” in the product literature); Below this are two adjacent push buttons; That button on the left is white, set in a black ring, and is marked “1 SEC” (called the “twitch frequency button” in the product literature); That button on the right is red, set in a black ring, and is marked “TETANUS”; Below this the unit is marked: [Unicorn logo] [new line] WELLCOME [new line] PERIPHERAL [new line] NERVE [new line] STIMULATOR”; Below this an adhesive label reads: “Use 5 ft. above floor level”;
Note Type: Acquisition
Notes: Gift of Ms. Dana Stetson.
Note Type: Historical
Notes: For general anesthesia, muscle relaxants are used to keep the entire body still during surgery. Muscle relaxants have also been used to control the muscle spasms caused by tetanus. Peripheral nerve stimulators (PNS) are used to determine the extent to which the drug has taken effect. They are used to stimulate a motor nerve with a mild electrical current, delivered through electrodes or needles, causing the related muscles to contract. The degree of this response is the most accurate guide to the extent of relaxation, called neuromuscular blockade.
The British pharmaceutical film Burroughs, Wellcome and Co. was founded in 1880. The company’s first overseas branch was established in Australia in 1886. In 1964, Burroughs Wellcome introduced its first PNS device, the “Block-Aid Monitor”, which was made in Tuckahoe, New York. The company’s next PNS device was the battery-powered “Wellcome Peripheral Nerve Stimulator”. The Wellcome PNS was made in Australia, and had been introduced by 1968.
The Wellcome PNS was equipped with two types of electrodes. The first was a pair of needle electrodes that were inserted under that patient’s skin and taped in place for continuous use throughout the surgery. The needle electrodes were connected to the device by a long electrical cord. The second type consisted of a pair of probes that were simply touched to the patient’s skin. The attachment that holds the probes was plugged directly into the device. These probes were meant only for intermittent use, such as during surgery, in the recovery room, or for the treatment of tetanus. Clinical use of the Wellcome Peripheral Nerve Stimulator continued into the 1980s, despite a 1974 report by Lippmann and Fields that the electrodes could cause burns.
Note Type: Publication
Notes: [New preparations and appliances]. South African Medical Journal. August, 1968;42(31):824.
Note Type: Exhibition
Notes: Selected for the WLM website.