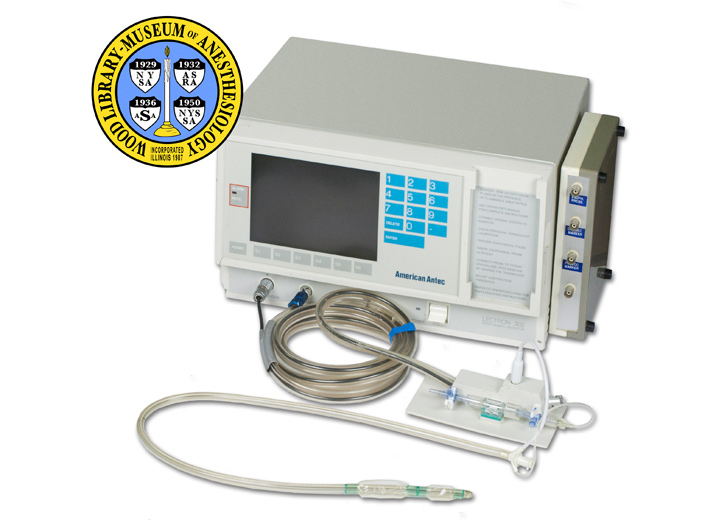
Lectron 302 Esophageal Monitor
The Lectron 302 Esophageal Monitor was one of the first esophageal pressure monitors designed specifically to aid the anesthesiologist in determining depth of anesthesia. When administering general anesthesia, the anesthesiologist must carefully balance two goals: prevent the patient from becoming conscious during surgery, and administer no more anesthetic than necessary. This requires great skill because the effects of anesthetics can vary from one individual to the next.
The aim of the esophageal monitor was to measure changes in muscle contractions of the esophagus due to anesthetics. A probe, or catheter, was inserted into the patient’s esophagus. Spontaneous esophageal muscle contractions, and contractions provoked by the anesthesiologist, were detected by a pressure transducer that delivered signals to the monitor. Unfortunately, the readings could be difficult to interpret, and eventually a number of studies brought the usefulness of esophageal monitoring into question. John M. Evans and Colin C. Wise, the inventors, and Antec Systems Limited, the assignee, applied to patent this monitor in 1981. The patent was granted in 1985.
Catalog Record: Lectron 302 Esophageal Monitor
Access Key: aikf
Accession No.: 1999-08-05-1 A
Title: Lectron 302 esophageal monitor.
Author: Evans, John M.
Author: Wise, Colin C.
Corporate Author: Antec Systems Limited.
Title variation: Alt Title
Title: Lectron 302 esophageal contractility monitor.
Title variation: Alt Title
Title: Lectron 302 oesophageal monitor.
Publisher: England : [Antec Systems Limited?] ; Valencia, Calif. : made for American Antec, [1985-1999].
Physical Description: 1 monitor : plastics, metals, paint ; 22 x 12.5 x 26 cm. + probe.
Subject: Monitoring, Intraoperative.
Subject: Esophageal Monitors.
Subject: Monitors.
Subject: Awareness.
Subject: Intraoperative Complications – prevention & control.
Note Type: General
Notes: Title from manufacturer’s marking on face of monitor.
Note Type: With
Notes: With an American Antec Esophageal Probe.
Note Type: Citation
Notes: Evans, JM, Wise, CC, inventors; Antec Systems Limited, assignee. Patient
monitoring equipment, prove for use therewith, and method of measuring
anesthesia based on oesophageal contractions. US patent March 5, 1985;
Application filed October 23, 1981.
Note Type: Citation
Notes: Isaac, PA. Lower oesophageal contractility and depth of anaesthesia.
Baillière’s Clinical Anaesthesiology. 1989;3(3):533-545.
Note Type: Physical Description
Notes: A stand alone esophageal contractility monitor; The shell of the monitor is a
light beige color; A digital display screen occupies most of the left side of
the face of the monitor; To the left (when facing the monitor) of the screen
is a red small light; “ALARM” is marked above the light and “MUTE” below the
light; Running below the screen are seven flat grey buttons marked, in order,
“ASSIST”, “S1”, “S2”, “S3”, “S4”, “S5”, “S6”; To the right of the screen is a
numbered key pad in five rows; “ENTER” is the only button in the fifth row;
“American Antec” appears in the white space below the key pad; On the right
side of the face of the monitor are basic instructions: “SEE OPERATOR’S
MANUAL FOR COMPLETE INSTRUCTIONS. [new paragraph] CONNECT PATIENT STATION TO
MONITOR. [new paragraph] CHECK PRESSURE TRANSDUCER CALIBRATION…”; Along the
lower edge of the monitor’s face are three ports; Running left to right they
are marked, “TRANSDUCER”, “PROVOCATION”, and “STETHOSCOPE”; The power switch
with a small light is to the right of the plugs; On the far right of the
lower edge are manufacturer’s markings: “LECTRON 302 [new line, in smaller
letters] esophageal monitor”; Taped to the top of the unit is a piece of
paper with user created, typed instructions for preparing and calibrating the
esophageal probe; A black electric cord with a 3 pronged plug exits a port at
the back of the monitor; Also at the back of the monitor are ports for “Fuses
1.5 AT”, printer, “RS 232” and a chart recorder; Manufacturer’s markings on
the back of the monitor include: “Model no. Lectron 302 Serial no. 0203 [new
line] Made in England for American Antec, Valencia, CA”.
Note Type: Reproduction
Notes: Photographed by Mr. William Lyle, 7/15/2010.
Note Type: Copyright
Notes: Patent: Evans, JM, Wise, CC, inventors; Antec Systems Limited, assignee.
Patient monitoring equipment, prove for use therewith, and method of
measuring anesthesia based on oesophagal contractions. US patent March 5,
1985; Application filed October 23, 1981.
Note Type: Acquisition
Notes: Donated to the WLM by Carl Rosow, M.D. Donation facilitated by Elliott V.
Miller, M.D.
Note Type: Historical
Notes: Lectron 302 esophageal monitor was one of the first (if not the first)
esophageal pressure monitors designed specifically to aid the
anesthesiologist in determining depth of anesthesia. As the designers of this
monitor explain in their patent application, the effects of anesthetics can
be unpredictable as patients’ responses vary considerably (Evans & Wise,
1985). Anesthesiologists strive to obtain an optimal depth of anesthesia. A
primary goal is to prevent any patient awareness during surgery while not
administering more anesthetics than necessary. The aim of the monitor was to
measure changes in esophageal contractility. A probe, or catheter, was
inserted into the patient’s esophagus. Esophageal contractions could be
stimulated by the inflation of a balloon in the catheter. The provoked, as
well as spontaneous, muscle contractions were detected by a pressure
transducer that delivered signals to the monitor. The monitor would display
the esophageal contractility index (ECI), which reflected the frequency of
spontaneous contractions and the amplitude of provoked contractions (Isaac,
1989). The readings could be difficult to interpret, and eventually a number
of studies brought their usefulness into question. At this time (August,
2010) esophageal monitors are no longer manufactured for this purpose.