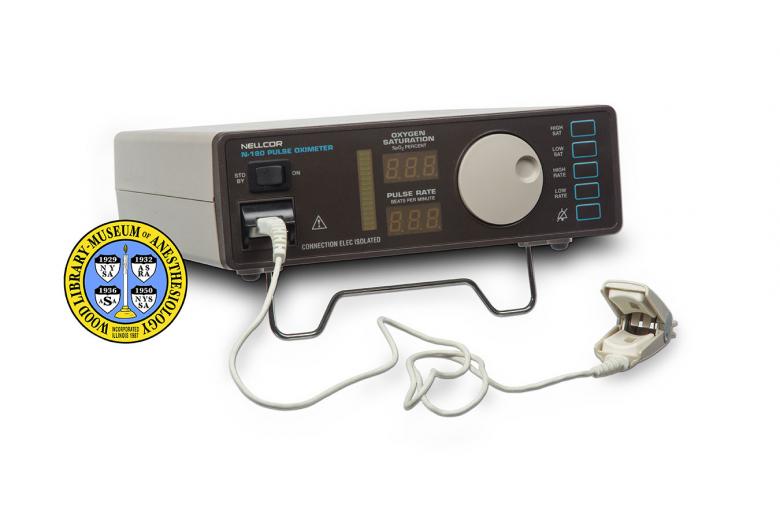
Nellcor Pulse Oximeter
Pulse oximeters revolutionized how anesthesiologists ensure that their patients get enough oxygen. Before a practical pulse oximeter was developed, physicians had to use expensive and time-consuming methods that posed adverse risks for the patient. With no skin puncture necessary, pulse oximeters use fast pulsing wavelengths of light to detect and calculate what percentage of a patient's oxygen-transporting hemoglobin is loaded with oxygen. The technical name for this measurement is arterial oxyhemoglobin saturation, abbreviated SpO2.
Like most modern medical devices pulse oximeters represent advancements in technology and a culmination of years of knowledge in basic and clinical sciences gained by the trial and error of numerous researchers. Japanese researcher, Takuo Aoyagi is often recognized for putting the "pulse" in pulse oximetry by using the waveform produced by the arterial pulse to measure and calculate SpO2. He first reported on the work of his team in 1974.
In 1981, anesthesiologist William New and two colleagues formed a new company called Nellcor. They released their first pulse oximeter, called the Nellcor N-100, in 1983. It quickly became a favored device. The N-100 was not only accurate and relatively portable, it incorporated a feature brand new to pulse oximetry technology, specifically, a sound indicator that reflected the rate of the pulse as well as the SpO2. If the SpO2 changed so did the pitch of the pulsing sound. Nellcor released the N-180, pictured here, around 1987.
Catalog Record: Nellcor Pulse Oximeter
Access Key: akhc
Accession No.: 2000-07-31-2 G
Title: Beckman D2 oxygen analyzer / Beckman Instruments, Inc.
Author: Pauling, Linus Carl, 1901-1994.
Author: Beckman, Arnold O. (Orville), 1900-2004.
Corporate Author: Beckman Instruments, Inc.
Title variation: Alt Title
Title: Beckman oxygen analyzer D2.
Title variation: Alt Title
Title: Beckman model D2 portable oxygen analyzer.
Title variation: Alt Title
Title: Oxygen meter.
Publisher: Irvine, CA : Beckman Instruments, Inc., [1956-1980].
Physical Descript: 1 monitoring device : metals, plastic, glass, rubber ; 13 x 15.5 x 21.5 cm.
Subject: Oxygen – analysis.
Subject: Monitoring – instrumentation.
Subject: Pauling, Linus Carl, 1901-1994.
Subject: Beckman, Arnold O. (Orville), 1900-2004.
Note Type: General
Notes: Years in the range for the possible year of manufacture (1956-1980) based on
the date of publications (cataloges and textbooks) with references to the
Beckman Model D2 Oxygen Analyzer. The early date is specifically based on two
1956 publications (Modern Power & Engineering, volume 50, page 112;
Combustion, volume 28, page 68) with advertisements for the “new” model D2.
Note Type: Citation
Notes: Fifty years of Beckman instruments. Eng Sci. 1986;49(5):20-27.
Note Type: Citation
Notes: Hager T. Linus Pauling: And the Chemistry of Life. Oxford: Oxford University
Press; 1998.
Note Type: Citation
Notes: Oxygen meter. The Scientific War Work of Linus C. Pauling website.
https://osulibrary.oregonstate.
edu/specialcollections/coll/pauling/war/narrative/page16.html. Accessed April
17, 2013.
Note Type: Citation
Notes: Simoni RD, Hill RL, Vaughan M, Tabor H. A classic instrument: The Beckman DU
Spectrophotometer and its inventor, Arnold O. Beckman. J Biol Chem.
2003;278(49):79-81.
Note Type: Citation
Notes: Thackray A, Myers M Jr. Arnold O. Beckman: One Hundred Years of Excellence.
Philadelphia: Chemical Heritage Foundation; 2000:180-186.
Note Type: Physical Description
Notes: One paramagnetic oxygen analyzer; The exterior of the device is a grey, metal
rectangular box, with a chrome plated handle and a push-button on top; Text
printed to the right of the push-button includes, “DANGER POSSIBLE EXPLOSION
HAZARD IF USED IN THE PRESENCE OF FLAMMABLE ANESTHETICS OR IN OTHER HAZARDOUS
LOCATIONS AS DEFINED IN THE NATIONAL ELECTRICAL CODE. REFER SERVICING TO
QUALIFIED SERVICE PERSONNEL.”; In the top, center of the front of the box is
a clear plastic window through which a scale is visible; The scale runs
horizontally; The upper part of the scale is marked in increments of
millimeters of mercury, from 0 to 750; The lower part of the scale is marked
in percentages from 0 to 100; Printed on the front of the box in black
lettering is, “BECKMAN®”, and to the right of this, “Model D2 Oxygen
Analyzer”; Secured to the back of the box a 3.5 cm wide strip of metal that
is bent into a horse shoe shape; Below this is a clear plastic tube that is
approximately 2.8 cm in diameter and 8 cm long; A rubber cap is secured to
each end of the tube; A thin grey tube is inserted into a small hole in the
center of the rubber cap at one end of the tube, and a thin clear tube is
inserted into a small hole in the cap at the other end of the rube; A sticker
secured to the bottom of the box is marked with the following text, “VOLTS 3”
“AMPS 0.7”, “MODEL D2”, “CAT NO 115583”, “HERTZ DC”, “SLR 0340737”,
“BECKMAN® INSTRUMENTS, INC.”, “MADE IN U.S.A. MARCA RES”, “SCIENTIFIC
INSTRUMENTS DIVISION”, “CAMPUS DR. AT JAMBOREE BLVD., IRVINE, CA 92713”.
Note Type: Reproduction
Notes: Photographed by Mr. Steve Donisch on January 14, 2013.
Note Type: Historical
Notes: Shortly before the United States entered WW II, the National Defense Research
Committee (NDRC) was created to facilitate scientific research that would
help the U.S. prepare for war. Scientists from varied specializations and
settings were recruited, including chemist Linus C. Pauling, PhD. Dr.
Pauling joined the NDRC on September 23, 1940. He was selected for a division
assigned to develop solutions for propellant and chemical related problems.
On October 3, 1940 he learned of the need for a means of measuring the
partial pressure of oxygen in closed environments, such as airplane cockpits
or submarines. By the first of November, Dr. Pauling and an assistant
researcher, Reuben Wood, developed a working prototype based on work
completed by French physicist Charles-Augustin de Coulomb in 1777. When
compared with other gases in the air we breathe, oxygen is unique as it is
attracted to magnetic fields, a property referred to as, “paramagnetic”. This
property was used to construct a device that employed magnets to measure the
proportion of oxygen in air samples.
Note Type: Historical
Notes: Subsequent prototypes of the Pauling oxygen meter, built to withstand
turbulent conditions, were successful; however Dr. Pauling’s lab was unable
to supply, in a timely manner, the large number of analyzers requested by the
government. For help with this Dr. Pauling turned to a fellow Caltech chemist
Arnold O. Beckman, PhD. During the 1930s, Dr. Beckman invented the first
commercially profitable electronic pH meter and collaborated on the
establishment of a manufacturing firm (National Technical Laboratories
Company), which built his pH meter and, beginning in 1940, his
spectrophotometer. Dr. Beckman agreed to manufacture Dr. Pauling’s oxygen
analyzer, and added his own innovations in order to improve efficiency and
better control quality. Due to issues related to the classified status of the
device, Dr. Beckman formed a separate manufacturing company to produce the
oxygen meters, Arnold O. Beckman, Inc. The oxygen analyzers produced by Drs.
Pauling and Beckman saved a significant number of lives not only during the
war, but afterward as well.
Note Type: Historical
Notes: Starting from Dr. Pauling’s design, Dr. Beckman and his team developed a
number of oxygen analyzers for a range of purposes and settings, including
military, industrial, and laboratory settings. The Model D2 described here
was introduced in 1956. It succeeded the Model D which was originally
designed for submarines. An anesthesiologist in Pasadena, California first
used a Beckman Oxygen Analyzer in the clinical setting to ensure that his
granddaughter had enough oxygen in her incubator. Oxygen concentrations that
are too high can also cause health problems, and by the late 1950s the device
was often employed to monitor oxygen levels in incubators.
Note Type: Publication
Notes: Barankin B. The impact of Linus Pauling on modern medicine and society. Bull
Anesth Hist. 1999;17(4):15-16.
Note Type: Publication
Notes: National historic chemical landmark: development of the Beckman pH Meter.
ACS: American Chemical Society website. https://portal.acs.
org/portal/acs/corg/content?_nfpb=true&_pageLabel=PP_SUPERARTICLE&node_id=718
use_sec=false&sec_url_var=region1&__uuid=e8f24c25-f3b9-461f-929e-20c3948801c3
Accessed Mary 3, 2013.
Note Type: Exhibition
Notes: Chosen for the WLM website (noted February 26, 2013).